Abstract
Introduction: Cutaneous T- cell lymphoma (CTCL) is a rare form of non-Hodgkin lymphoma. Patients with CTCL suffer reduced quality of life from intractable itching and recurrent infections. Advanced stages have a poor prognosis. Mogamulizumab (Moga) is a monoclonal antibody directed against chemokine receptor 4 (CCR4), which is overexpressed on malignant T-cells. In a Phase I-II study in CTCL, Moga demonstrated a tolerable safety profile with a 37% overall response rate (ORR). Based on these results, MAVORIC [NCT01728805], an open-label, multinational, randomized, Phase III study, was initiated to compare Moga to vorinostat (Vor) in previously treated CTCL. This study is the largest randomized trial and the first pivotal trial to use progression-free survival (PFS) as a primary endpoint in CTCL.
Methods: Adult patients with histologically confirmed mycosis fungoides (MF) or Sézary syndrome (SS) who had failed ≥1 systemic therapy were enrolled, stratified by disease type (MF or SS) and stage (IB/II or III/IV), and randomized 1:1 to Moga 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or Vor (400 mg daily). Patients randomized to Vor could crossover to Moga upon progression or intolerable toxicity. The primary endpoint was investigator-assessed PFS in the randomized population using the global composite response (based on skin, blood, nodes and viscera) according to the ISCL/EORTC consensus guidelines. Sample size was calculated to provide 90% power to detect a 50% improvement in PFS. Key secondary endpoints included ORR, duration of response (DOR) and quality of life (QoL).
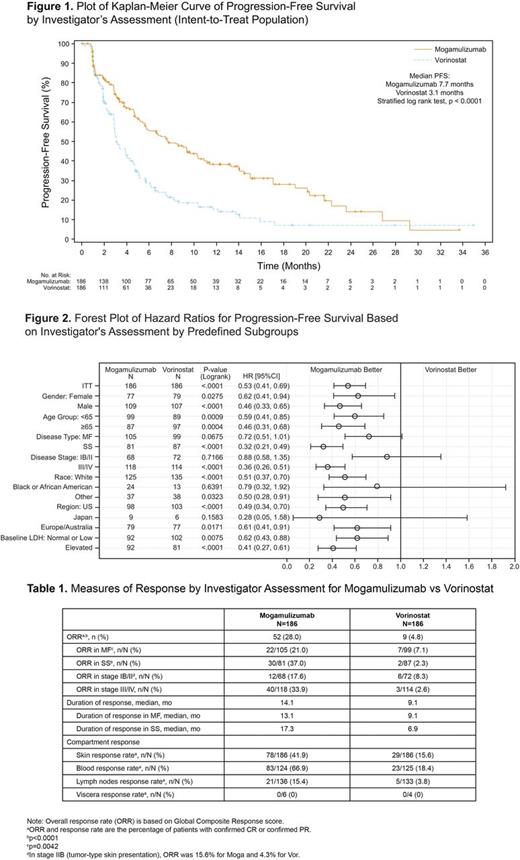
Results: A total of 372 patients were randomized (Intent-to-Treat population) and had the following characteristics (Moga vs Vor): median age 63.5 yrs (25-101) vs 65.0 yrs (25-89); ECOG-PS 0-1, 184 (99%) vs 186 (100%); ECOG-PS 2, 2 (1%) vs 0; stage IB/IIA, 36 (19.4%) vs 49 (26.3%); stage IIB, 32 (17.2%) vs 23 (12.4%); stage III/IV, 118 (63.4%) vs 114 (61.3%); MF, 105 (56.5%) vs 99 (53.2%); SS, 81 (43.5%) vs 87 (46.8%). The median number of prior systemic treatments for both the Moga and Vor arms was 3. According to investigator assessment, treatment with Moga resulted in a significant improvement in PFS compared to Vor (HR 0.53 [95% CI: 0.41, 0.69], p<0.0001) with a median PFS of 7.7 months (95% CI: 5.7, 10.3) for Moga and 3.1 months (95% CI: 2.9, 4.1) for Vor (Figure 1). Improvement in PFS was also demonstrated based on independent review (HR 0.64 [95% CI: 0.49, 0.84], p=0.0007): 6.7 months for Moga and 3.8 months for Vor. Moga was associated with superior PFS in predefined subgroups (Figure 2). Global ORR was significantly improved in the patients randomized to Moga at 28.0% vs 4.8% for Vor (p<0.0001). ORR in predefined subgroups, DOR and response by disease compartment all favored Moga vs Vor (Table 1). Significant improvement in ORR was found with Moga vs Vor in patients with both MF (21.0% vs 7.1%, respectively; p=0.0042) and SS (37.0% vs 2.3%, respectively; p<0.0001). An ORR of 30.1% was observed in Moga treated patients who crossed over from Vor. Patient-reported outcomes, as measured by the Skindex-29 and FACT-G, showed significantly greater symptom reduction and improved functional status in favor of Moga vs Vor in early cycles and throughout treatment (p<0.05). The median dose intensity for Moga was 97.5% vs 95.7% for Vor, supporting adequate treatment in both arms. Treatment exposure was longer with Moga (median 170 vs 84 days for Vor). The most common treatment-emergent adverse events (TEAEs; >20%) that were more frequent (>15% difference) in the Moga vs Vor arm included infusion-related reactions (33.2% vs 0.5%, respectively) and skin eruptions due to drug (23.9% vs 0.5%, respectively). The majority of TEAEs with Moga were mild to moderate in severity (grade I/II, 54.9%; grade III/IV/V, 42.4%). Common TEAEs reported more often with Vor vs Moga included diarrhea (61.8% vs 23.4%), nausea (42.5% vs 15.2%), thrombocytopenia (30.6% vs 11.4%), dysgeusia (29.0% vs 3.3%), and increased blood creatinine (28.0% vs 3.3%).
Conclusions: In this first report of a randomized Phase III study evaluating PFS as primary endpoint in CTCL, Moga, a novel CCR4-targeting antibody therapy, demonstrated significantly superior PFS, ORR, and QoL compared to Vor in patients with previously treated CTCL. The safety profile was consistent with previous reports. This study supports Moga as a valuable new therapeutic option in patients with CTCL.
Kim: Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; Soligenix: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Consultancy, Research Funding; Neumedicine: Research Funding; miRagen: Research Funding; Merck: Research Funding; Medivir: Membership on an entity's Board of Directors or advisory committees; Kyowa-Kirin-Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innate Pharma: Consultancy, Research Funding; Horizon Pharma: Consultancy, Research Funding; Forty Seven Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tetralogic: Research Funding; Millennium Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees. Bagot: Innate Pharma: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Actelion: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Kyowa: Membership on an entity's Board of Directors or advisory committees. Horwitz: Kyowa-Hakka-Kirin: Consultancy, Research Funding; Mundipharma: Consultancy; ADCT Therapeutics: Research Funding; Forty-Seven: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; HUYA: Consultancy; Millenium/Takeda: Consultancy, Research Funding; BMS: Consultancy; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Aileron Therapeutics: Research Funding. Whittaker: Celgene: Honoraria; Galderma: Research Funding. Vermeer: Innate Pharma safety board for IPH4102-101: Membership on an entity's Board of Directors or advisory committees. Zinzani: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; J&J: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sokol: Spectrum Pharmaceuticals: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy. Kim: Kyowa Kirin Pharmaceutical Development, Inc.: Other: Clinical trials investigator; Solgenix: Other: Clinical trials investigator; Actelion: Consultancy; Cutaneous Lymphoma Foundation: Membership on an entity's Board of Directors or advisory committees; US Cutaneous Lymphoma Consortium: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy. Ortiz-Romero: ACTELION: Consultancy; 4SC: Consultancy; Innate Pharma: Consultancy; Takeda: Consultancy; MEDA: Research Funding. Eradat: Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding; Novartis: Research Funding; Celgene: Research Funding; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding. Scarisbrick: 4SC: Consultancy; Takeda: Consultancy; Mallinckrodt: Consultancy; Innate Pharma: Consultancy; Actelion: Consultancy. Elmets: NCI: Research Funding; Veterans Administration: Research Funding; California Wine Grape Assn: Research Funding; Solegenix: Research Funding; Idera: Research Funding; Elorac: Research Funding; Ferndale Labs: Consultancy, Research Funding; Astellas Pharma: Research Funding. Dalle: Kyowa Hakko Kirin Pharmaceutical: Research Funding. Fisher: Celgene: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Halwani: Amgen: Research Funding; Pharmacyclics: Research Funding; Takeda: Research Funding; Genetech Inc.: Research Funding; Roche/Genentech Inc.: Research Funding; Seattle Genetics: Research Funding; Bristol Myers Squib: Research Funding; Kyowa Hikko Kirin: Research Funding; AbbVie: Research Funding; Immune Design: Research Funding; Miragen: Research Funding. Poligone: Actelion Pharmaceutical: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy; Kyowa Hakko Kirin: Research Funding; Soligenix: Research Funding. Khot: Celgene: Consultancy; Janssen: Consultancy; Amgen: Other: Travel Grant. Moskowitz: Incyte: Research Funding; Takeda: Honoraria; Bristol Myers-Squibb: Consultancy, Research Funding; ADC Therapeutics: Research Funding; Seattle Genetics: Honoraria, Research Funding. Dwyer: Kyowa Kirin Pharmaceutical Development, Inc.: Employment. Moriya: Kyowa Kirin Pharmaceutical Development, Inc.: Employment. Humphrey: KYOWA KYRIN PHARMACEUTICAL DEVELOPMENT: Employment. Hudgens: Clinical Outcomes Solutions: Consultancy, Research Funding. Grebennik: Kyowa Kirin Pharmaceutical Development, Inc.: Employment. Tobinai: AbbVie: Research Funding; Chugai: Honoraria, Research Funding; Kyowa Hakko Kirin: Honoraria, Research Funding; Eisai: Honoraria, Research Funding; Zenyaku Kogyo: Honoraria; GlaxoSmithKline: Research Funding; Takeda: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Servier: Research Funding; Janssen: Honoraria, Research Funding; HUYA Bioscience: Honoraria; Daiichi Sankyo Co., Ltd: Consultancy, Honoraria; Mundipharma: Honoraria, Research Funding; Ono Pharmaceutical: Honoraria, Research Funding. Duvic: MDACC: Other: Safety Oversight Committee, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal